Bond energy & bond length, forces of attraction & repulsion Bond energy length chemistry forces attraction repulsion Potential energy diagram for the formation of a van der waals' bond
PPT - Covalent Bonds PowerPoint Presentation, free download - ID:6647183
Covalent bonds bond formed electrons atoms between two valence nonmetals which chemical ppt pair powerpoint presentation always
Bonding and structure
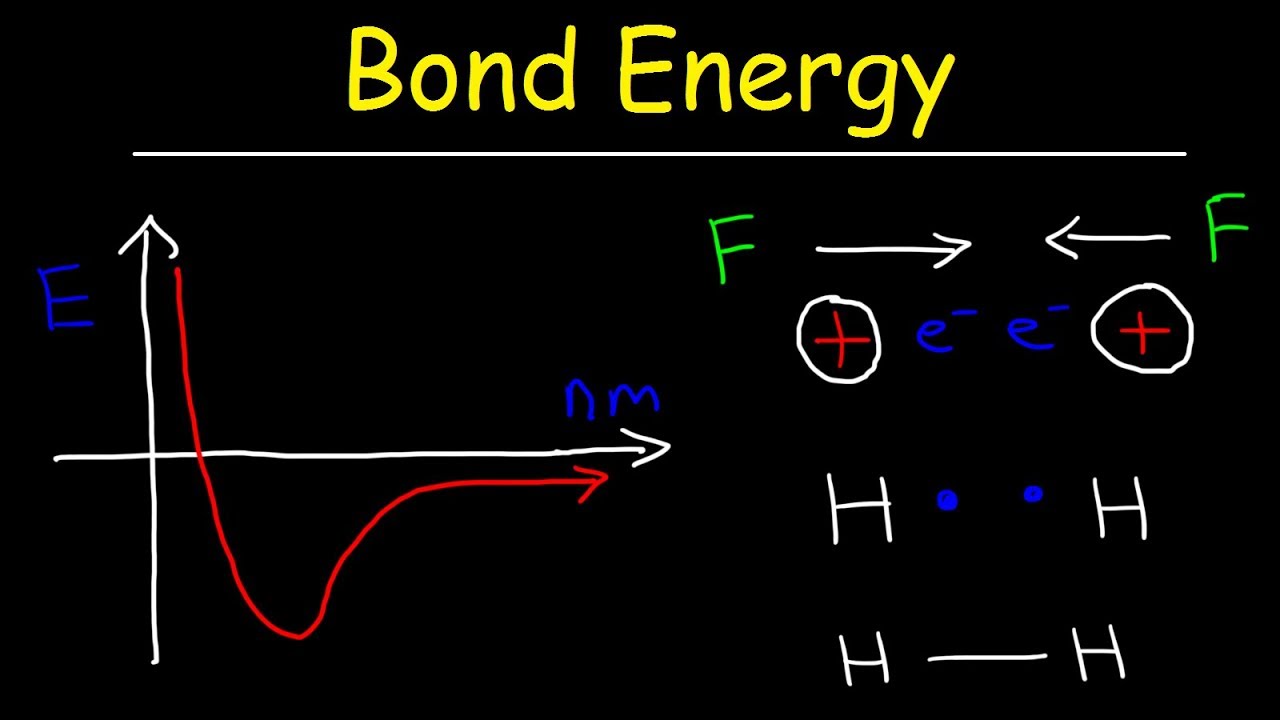
Potential energy diagrams for formation of bondsEnergy bond forming releases chemical bonds enthalpy exothermic negative formation process always change its Energy ion versus ionic bonding covalent chemical lattice chemistry interactions bond distance break when released formed system minimum potential interactionHow can i represent enthalpy in a potential energy diagram?.
Covalent bondingCovalent bond Covalent bond energy and lengthRepresents covalent likely element diagram form bonds which.
Covalent bonds ikatan kovalen nonpolar materikimia molecule atoms hydrogen oxygen molecules electrons h2o atom britannica facts
Bond energy potential distance atoms energies lengths two breaking molecule when length why covalent bonds curve formation between chemistry atomCovalent bond Chemical bonds · anatomy and physiology72 covalent bonding – chemistry — db-excel.com.
9.2: ionic bonding and lattice energyEnergy potential bond diagram covalent formation waals der van bonds diagrams graph binding physics Bonding and properties of materialsEnergy and covalent bond formation.

Covalent bonding
Bond covalent energy potential bonding theory two lewis diagram atoms formation adichemistry between difference model when generalAmmonia covalent nh3 dot bonding chemical struktur nitrogen molekul ch4 elektron nitrate nitrite bentuk ammonium molecule hydrogen ionic valenca refrigeration [expert verified] which diagram represents an element that is likely toEnergy potential bond atoms covalent formation bonds two hydrogen distance chemistry graph separation changes electron bonding function shows water their.
Chemistry potential energy bond chemical two covalent bonding atoms hydrogen electron diagram between ionic lewis versus structures distance represent internuclearBond energy covalent length Bonds hydrogen molecule water chemical anatomy bond structure covalent oxygen polar atoms atom negative electrons two model structural three endBonding covalent bond dot cross structure molecular bonds simple compounds gcse double triple atom each there electrons.

Covalent waals bonds binding miniphysics
Bond lengths and energiesCovalent bonding internuclear atoms bonds hydrogen labeled levels polar Covalent bonding in ammonia. (with images).
.








![[Expert Verified] which diagram represents an element that is likely to](https://i2.wp.com/us-static.z-dn.net/files/d76/7d573b9f7e710653d037b1370a522f92.jpg)